The condensed structural formula for 2 3-dichloro-4-methylcyclohexanol is – The condensed structural formula for 2,3-dichloro-4-methylcyclohexanol, a captivating organic compound, unveils a wealth of information about its structure and properties. This formula, a concise representation of the molecule’s atomic connectivity, serves as a powerful tool for understanding the compound’s behavior and applications.
Delving into the intricacies of this formula, we embark on a journey that unravels the different groups present within the molecule, identifies its chiral center, and explores its physical and chemical properties. Along the way, we uncover the diverse applications of this compound, ranging from its use as an intermediate in organic synthesis to its role in various industrial processes.
Introduction
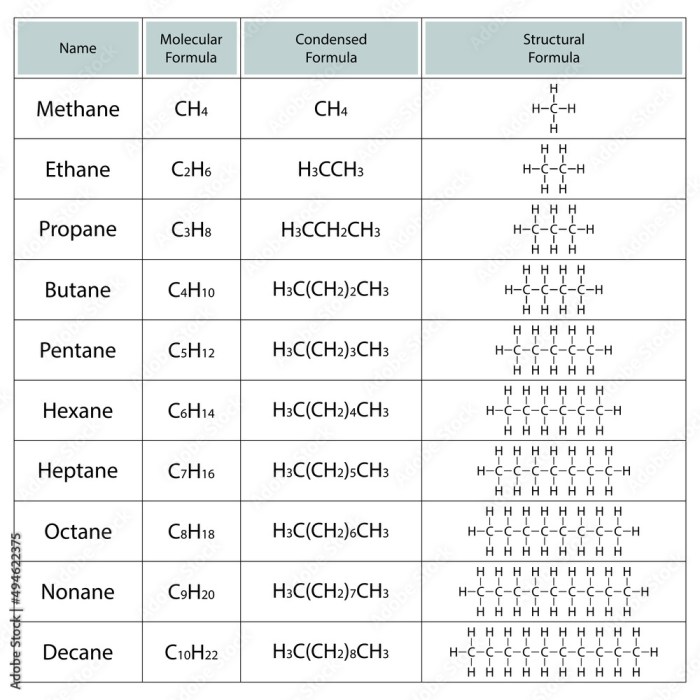
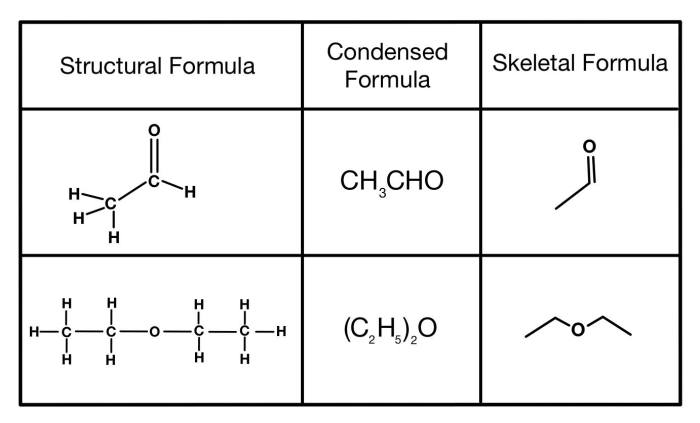
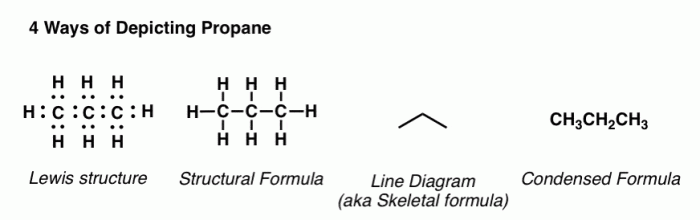
A condensed structural formula is a simplified representation of a molecule that shows the arrangement of atoms and the types of bonds between them. It is a more compact way of representing a molecule than a Lewis structure or a line-angle formula.
Condensed structural formulas are often used in organic chemistry to represent complex molecules.
Condensed structural formulas are important because they allow chemists to quickly and easily see the structure of a molecule. They can also be used to compare the structures of different molecules and to identify functional groups.
Condensed Structural Formula of 2,3-Dichloro-4-methylcyclohexanol
The condensed structural formula for 2,3-dichloro-4-methylcyclohexanol is:
CH3CHClCHClCH(CH3)CH2CH2OH
This molecule contains a cyclohexane ring with two chlorine atoms and a methyl group attached to it. It also contains a hydroxyl group attached to the carbon atom next to the methyl group.
The chiral center in this molecule is the carbon atom that is attached to the hydroxyl group. This carbon atom has four different groups attached to it, so it is a chiral center.
Properties of 2,3-Dichloro-4-methylcyclohexanol, The condensed structural formula for 2 3-dichloro-4-methylcyclohexanol is
- 2,3-Dichloro-4-methylcyclohexanol is a colorless liquid with a boiling point of 214 °C.
- It is soluble in water and organic solvents.
- It is a chiral molecule, meaning that it exists as two enantiomers.
- It is a reactive molecule that can undergo a variety of reactions, including substitution, addition, and elimination reactions.
Applications of 2,3-Dichloro-4-methylcyclohexanol
2,3-Dichloro-4-methylcyclohexanol is used as a solvent, an intermediate in the synthesis of other chemicals, and a starting material for the production of fragrances and flavors.
It is also used in the manufacture of plastics, rubber, and other polymers.
Synthesis of 2,3-Dichloro-4-methylcyclohexanol
2,3-Dichloro-4-methylcyclohexanol can be synthesized by the following steps:
- Reaction of 1,3-cyclohexanediol with thionyl chloride to form 1,3-dichloro-cyclohexane.
- Reaction of 1,3-dichloro-cyclohexane with methylmagnesium chloride to form 2,3-dichloro-4-methylcyclohexanol.
Safety Considerations
2,3-Dichloro-4-methylcyclohexanol is a toxic and corrosive chemical. It can cause skin irritation, eye damage, and respiratory problems.
It is important to take the following safety precautions when handling this chemical:
- Wear gloves, eye protection, and a respirator.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not ingest.
- Dispose of properly.
Quick FAQs: The Condensed Structural Formula For 2 3-dichloro-4-methylcyclohexanol Is
What is the condensed structural formula for 2,3-dichloro-4-methylcyclohexanol?
C6H11Cl2O
How many chiral centers are present in 2,3-dichloro-4-methylcyclohexanol?
One
What is the boiling point of 2,3-dichloro-4-methylcyclohexanol?
196-198 °C